Oxidation Field: A Newly-Discovered Component of Skin Chemistry

Skin chemistry: that vague, oft-cited reason for perfumes smelling different on different people.
But what does that term actually mean?
The Society of Scent defines it this way: “The chemical behavior of the skin varies from individual to individual and is influenced by variables such as diet, skin type, environment and medications.”
Essentially, fragrance molecules react with genetically, environmentally, and behaviorally determined facets of human skin. These reactions can change how a perfume smells on a particular person.
One major factor is the distribution of lipids (fats and oils) on your skin. These react with topically applied products such as fragrances in different ways, potentially altering the way they smell on you.
But why are these reactions occurring, and what components contribute to them? One surprising mechanism for such skin chemistry reactions was revealed in a new September 2022 article in Science.
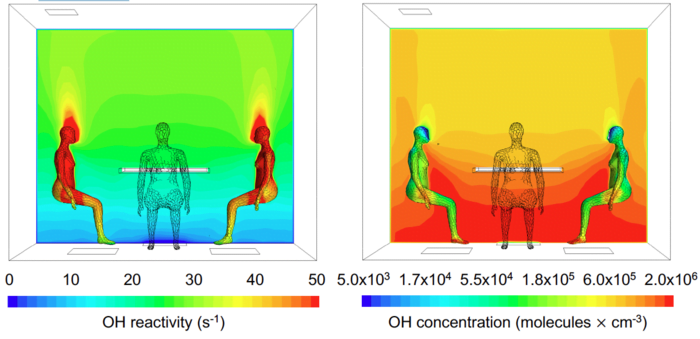
The research paper from the Max Planck Institute for Chemistry, “People Generate Their Own Oxidation Field and Change the Indoor Air Chemistry Around Them,” describes a rather staggering discovery: “High levels of hydroxyl radicals (OH) can be generated indoors, simply due to the presence of people and ozone.”
Put simply, the interaction of the natural oils on human skin and ozone appearing in the atmosphere creates chemicals called hydroxyl radicals. Because the entire human body is covered in these oils, and ozone is always present (at varying levels) in the Earth’s atmosphere, hydroxyl radicals are always hovering in a field around the human body. These can then potentially interact with substances around us, including topical products like perfumes.
Think of an aura, but instead of ~vibrations~ it’s hydroxyl radicals.
But what exactly is a hydroxyl radical?
Made up of hydrogen and oxygen, the hydroxyl radical is one of several kinds of free radicals. These are unstable molecules that are highly reactive and can damage cells. They can cause aging and a number of diseases. There’s lots we still don’t know about free radicals, how exactly they make people sick, and how this can be prevented.
If you’ve heard antioxidants are good for you, that’s because they counteract unstable free radical molecules, effectively neutralizing them. Free radicals, on the other hand, are “oxidants,” meaning they cause the chemical process called oxidation.
Rust is a classic example of oxidation, in which iron is oxidized and turns into brown iron oxide.
The discovery that human skin oils generate a field of hydroxyl radicals around us is certainly alarming from a health and safety standpoint. It also provides several fascinating new explanations for skin chemistry phenomena. Because levels of these skin oils may vary between individuals, and ozone level can vary widely depending on environmental factors, the interaction of these elements could provide widely variable levels of hydroxyl radicals.
Thus, both the varying levels of ozone and levels of hydroxyl radicals created by interaction with ozone might potentially interact with fragrance products in a way that influences how they smell to humans. “This oxidation field will also impact the chemical signals we emit and receive, and possibly help explain the finding that our sense of smell is generally more sensitive to molecules that react faster with OH,” says atmospheric chemist Jonathan Williams, the leader of the recent Max Planck Institute for Chemistry project.
If reaction with hydroxyl radicals makes aroma molecules easier to detect, that means that levels of these radicals and of ozone can have a significant impact on the way a perfume smells on a particular person on a particular day. Questions of fragrance projection, sillage, and longevity seem most immediately relevant, but it’s possible interactions with these chemicals can shape our perception of scents in other, less obvious ways as well.
For one thing, the amounts of different types of oil on people’s skin can vary widely. The Max Planck Institute for Chemistry study found that a compound called squalene, a triterpene, is especially prone to reacting with ozone. Making up about 10% of your skin oils, this compound keeps your skin soft and smooth and protects it from the elements. The other 90% is a combination of other oils, many of which are also in the triterpene category.
There are about 20,000 different oils called triterpenes. These are generally regarded as protective and often appear in supplements and medicines. Amounts of these on different people’s skin can vary. This is especially true because triterpenes are common in cosmetics and topical skin treatments, meaning people apply different levels of additional oils to their skin.
Even without human skin in the equation, many perfumes have triterpenes in them already, since this is a large category of naturally-occurring oils. Frankincense oil, for instance, contains triterpenes with anti-inflammatory properties. Even squalene itself, the triterpene found to be most reactive with ozone, is commonly used in skincare products as a moisturizer and could conceivably appear in perfume oils for this purpose.
These varying quantities of different oils on people’s skin, in their cosmetics, and in their perfumes add up. The result? Varying amount of skin oils that might alter the amount of hydroxyl radicals produced by exposure to ozone.
Another factor in the amount of hydroxyl radical production is ozone concentration. Different times and places can have varying levels of ozone in the air depending on a number of factors.
High ambient ozone concentrations can occur anywhere and at any time, but usually occur in the early afternoon, when the amount of sunlight is the most intense.
Additionally, although car traffic is the main source of ozone precursors (the chemical compounds that react to create ozone in the air around us), ozone concentrations are actually typically higher in rural areas than in cities.
Sometimes called the Ozone Paradox, this phenomenon is explained by the fact that car traffic also produces high levels of nitric oxide, which reacts with ozone and helps degrade and remove it. However, ozone that travels downwind from cities to rural areas and is created by countryside sunshine does not meet the same level of nitric oxide in the atmosphere, meaning it can take longer for rural ozone to dissipate.
Due to a number of human and environmental factors, such as season, weather, and human activities, ozone concentrations vary widely from day to day. A number of companies and government agencies produce daily ozone forecasts. If you’re in the US, for instance, you can check your location’s ozone level in the U.S. Air Quality Index. Ozone is an unstable gas that is harmful for humans to breathe, so it’s good to check in on the amount you’re exposed to.
All this is to say that from person to person and place to place, the amount of hydroxyl radicals produced on the skin will vary widely. The amount of these available to react with fragrance molecules could potentially be a major component of what we think of as skin chemistry.
Different fragrance materials have different levels of reactivity with hydroxyl. The higher the reactivity, the easier it generally is for humans to smell the material. Thus, that oxidation field, though concerning, likely plays a large role in the way scents are expressed on skin.
Of course, this discovery also raises concerns for consumer product safety. Right now, the safety of chemicals emitted by consumer products is usually being tested in isolation. Williams argues that tests should also be conducted in the presence of people and ozone, to ensure that products are still safe when encountering the oxidation field.
It will be interesting to see whether the regulatory environment around fragrances takes Williams’ concern to heart. Testing the interactions of aroma chemicals and hydroxyl radicals could teach us a lot about our sense of smell. Along the way, we might learn more about the specifics of skin chemistry, and the reactions that make perfumes smell different on different people.
This work might alert us to dangers we hadn’t previously been aware of. But it might also bring to light new opportunities for fragrance use to benefit health and safety. Researchers have already studied the potential of antioxidant-heavy fruit essential oil perfumes to combat free radicals in the air. A 2020 study by Deshinta et al. revealed that apple, orange, grape, melon, and lemon extracts all effectively decreased free radicals in the air at various concentrations.
There is much we have yet to learn about free radicals, including hydroxyl radicals, and their impact on human health. Studying interactions between these and fragrance materials might produce benefits for product safety, consumer health, and our understanding of how fragrances smell on people’s skin.









One thought on “Oxidation Field: A Newly-Discovered Component of Skin Chemistry”